How much liquid water is generated in a fuel tank due to condensation? This is an engineering analysis focused on aviation, where water in the fuel tanks is a known cause of accidents. There is plenty of anecdotal evidence and opinions ranging from “Never happens” to “Critical in humid areas”. At the end of this note, you will be able to understand the physics and quantify the generation rate. Let’s get started.
Summary
- Nearly empty light-aircraft tanks in extreme hot and humid environments with extreme temperature swings theoretically could condense approximately a couple of fluid ounces a week.
- The generation rate linearly scales with empty tank volume and humidity, but exponentially with temperature.
- Normally vented tanks substantially reduce the water influx rate, but do have a breathing mode that can pump moist air during temperature and pressure swings.
- Condensation is more likely to be a long term storage threat; Large volumes of water are more likely to be ingress of liquid water.
Dry & Wet Air
Water is a key enabler of life and dramatically affects the behavior of air. We call “dry air” the mixture of mostly nitrogen (80%), oxygen (20%), and trace other constituents (Ar, carbon dioxide, etc). “Wet air” is what we normally encounter and is dry air + water. “Air” could also include particles + bugs + dirt. You can learn more at my course notes here for an introduction and here for non-standard atmospheres.
The important takeaways are:
- Adding water decreases air density since water has a lower molecular mass than air. (Technical note: Water has 2 Hydrogen of mass 1 plus 1 Oxygen of mass 16 for a total of 18. Air on the other hand has 80% diatomic Nitrogen of mass 28 and 20% diatomic Oxygen at mass 32 for a total of 28.97.).
- Increasing temperature substantially increases the absolute water carrying capacity of air. Water vapor at 100% relative humidity consists of 0.7% of the wet air mass at 50 F and 6% at 110 F, but a whopping 15% at 140 F. This is why pilots need to be much more concerned with high humidity at high temperatures and not so much at lower temperatures (This is in addition to the temperature effects on density altitude).
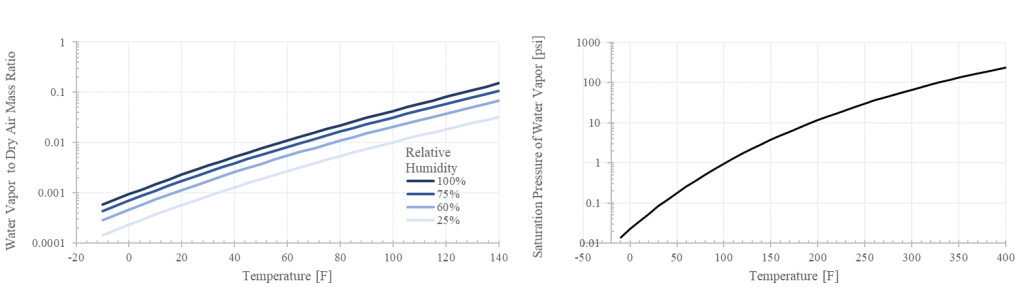
The saturation pressure (Ps) in Figure 2 is generated from the Arden-Buck approximation as an exponential function of temperature. This also indicates the pressure at which boiling occurs (e.g. 212 F at sea level pressures of 14.69 psi; and 200 F at 10000 ft with a pressure of approximately 10 psi).
Design a worst case scenario
Let’s pick a scenario where a nearly empty 25 gallon tank completely condenses the water vapor. Plus there is a complete air exchange/recharge of hot and humid (120 F and 100% relative humidity) air once per day. How much water is generated?
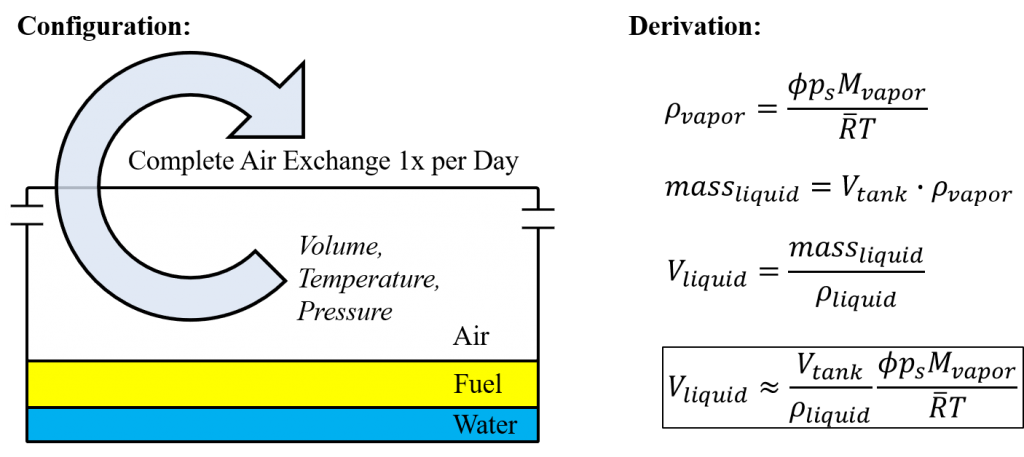
The answer is about 0.25 oz (7.5 ml) per day. Consider it one cupped-hand of water, or about a 2.5″ spot of water, or 0.75 seconds of fuel at 10 gal/hr. That’s enough to fully grab your attention.
The rate scales linearly with the tank’s air volume so keeping the tank 90% full reduces the generation rate by 90%. Being in 30% humidity air reduces the rate by 70%. Doubling the elapsed days doubles the generated volume. Notice that the rate exponentially scales with temperature (ps/T is exponential).
Critically, we can bound the generation rate. For example, at 120F and 100% humidity with a 20 gallon tank, the rate should be less than 0.20 oz per day.
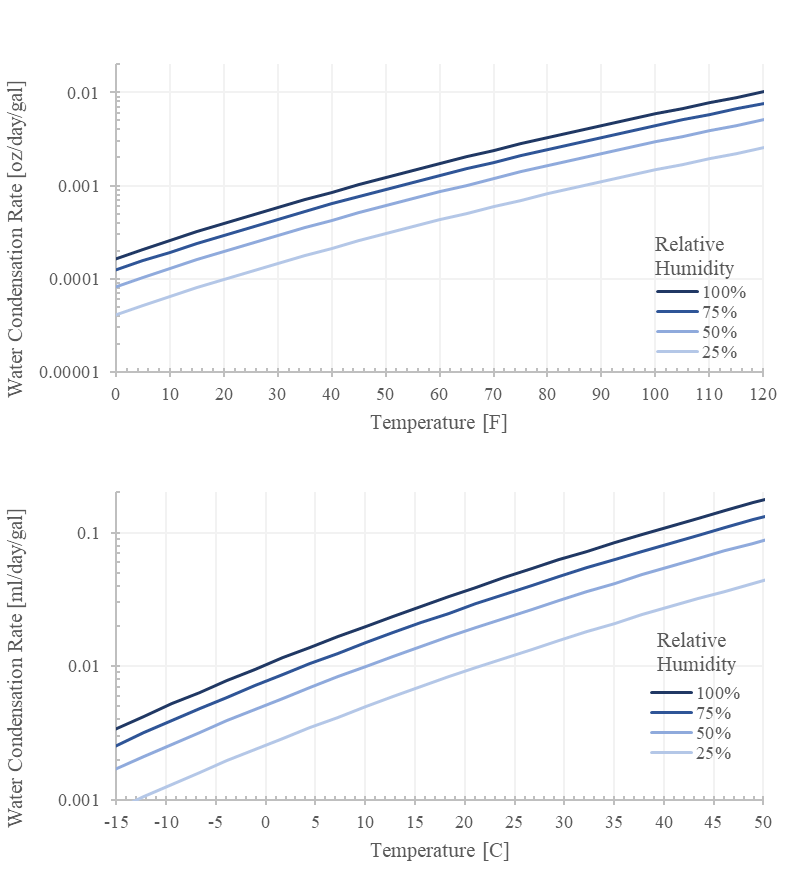
If you are generating more than this, chances are extremely likely that there is another mechanism responsible. Go find it.
Maximum Condensing Case with an more Realistically Vented Tank
To be continued
Cold Soak & Descent
To be continued

